- Acumen Powered by Robins Kaplan LLP®
- Affirmative Recovery
- American Indian Law and Policy
- Antitrust and Trade Regulation
- Appellate Advocacy and Guidance
- Business Litigation
- Civil Rights and Police Misconduct
- Class Action Litigation
- Commercial/Project Finance and Real Estate
- Corporate Governance and Special Situations
- Corporate Restructuring and Bankruptcy
- Domestic and International Arbitration
- Entertainment and Media Litigation
- Health Care Litigation
- Insurance and Catastrophic Loss
- Intellectual Property and Technology Litigation
- Mass Tort Attorneys
- Medical Malpractice Attorneys
- Personal Injury Attorneys
- Telecommunications Litigation and Arbitration
- Wealth Planning, Administration, and Fiduciary Disputes
Acumen Powered by Robins Kaplan LLP®
Ediscovery, Applied Science and Economics, and Litigation Support Solutions
-
April 23, 2024David Martinez Recognized Among Top 100 Lawyers in Los Angeles by LA Business Journal
-
April 15, 2024Robins Kaplan Named to 2024 BTI Client Service A-Team
-
April 9, 2024Robins Kaplan LLP Files Complaint Against Social Media Giants Meta, Snap, TikTok on Behalf of Spirit Lake Nation, Menominee Indian Tribe of Wisconsin
-
April 24, 2024IP Leadership Executive Summit
-
April 24, 2024IP Odyssey: Navigating the Latest Developments in Intellectual Property Law
-
April 30, 2024Navigating Generational Dynamics
-
March 2024e-Commerce: Pitfalls and Protections
-
March 22, 2024‘In re Cellect’:
-
March 14, 2024How Many Cases Have You Tried to a Verdict?
-
September 16, 2022Uber Company Systems Compromised by Widespread Cyber Hack
-
September 15, 2022US Averts Rail Workers Strike With Last-Minute Tentative Deal
-
September 14, 2022Hotter-Than-Expected August Inflation Prompts Massive Wall Street Selloff
Find additional firm contact information for press inquiries.
Find resources to help navigate legal and business complexities.
Exactech® Knee and Hip Recall Lawsuit
You must read the following notice before sending an e-mail message to Robins Kaplan LLP.
Any information that you send us in an e-mail message should not be confidential or otherwise privileged information. Sending us an e-mail message will not make you a client of Robins Kaplan LLP. We do not accept representation until we have had an opportunity to evaluate your matter, including but not limited to an ethical evaluation of whether we are in a conflict position to represent you. Accordingly, the information you provide to us in an e-mail should not be information for which you would have an expectation of confidentiality.
If you are interested in having us represent you, you should call us so we can determine whether the matter is one for which we are willing or able to accept professional responsibility. We will not make this determination by e-mail communication. The telephone numbers and addresses for our offices are listed on this page. We reserve the right to decline any representation. We may be required to decline representation if it would create a conflict of interest with our other clients.
By accepting these terms, you are confirming that you have read and understood this important notice.
Have you received a letter from your surgeon informing you of a recall regarding your Exactech® knee and hip replacement device? You may be entitled to additional compensation including possible claims to help recover medical costs.
OUR LAWYERS ARE READY TO ASSIST YOU.
Robins Kaplan LLP is investigating the device recall of Exactech polyethylene inserts that were used in knee and hip arthoplasty. If you have a knee polyethylene insert included in this recall, and your surgeon contacts you about follow up, please contact us at 1.800.811.9972 for a free evaluation.
Patients Who Were Implanted with an Exactech Recalled Device
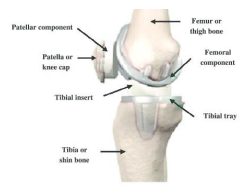
In August 2021, Exactech recalled its first product due to these defective plastic inserts. Now Exactech has expanded the recall to include the remaining 55,269 non-conforming Exactech Knee Ultra-High Molecular Weight Polyethylene inserts in the field, regardless of shelf life. Exactech estimates that there are approximately a total of 147,732 inserts implanted in the US since 2004 that were produced with non-conforming packaging. The Food and Drug Administration (FDA) classified the recall as a Class II recall, which occurs when consumers may experience severe health consequences of a temporary or medically reversible nature.
As part of the expanded recall, Exactech provided surgeons with a draft letter to their patients who were implanted with Exactech knee devices packaged in non-conforming bags. It was recommended that surgeons customize this letter and send it to patients implanted with non-conforming devices.
Additionally, Exactech provided patients and physicians with:
- A list of all the surgeons’ knee patients who received defective non-conforming bags;
- Frequently asked questions page available online and
- A tool on Exactech’s website that allows a patient to enter her/his implant serial number and confirm whether or not that implanted device is non-conforming.
Exactech has also published an online list of affected devices, product codes, descriptions, and serial numbers. Recalled devices include the following Exactech knee systems:
- Optetrak: 60,926 implanted units since 2004
- Optetrak Logic: 60,518 implanted units since 2004
- Truliant Knee Replacement: 24,727 implanted units since 2004
Injuries Potentially Related to the Exactech Knee Device Recalls
The defect contained within these Exactech knee and hip devices can lead to the following injuries:
- Premature wear of the device
- Bone loss
- Disintegration of bone cells (lysis)
- Pain
- Loosening
- Component fatigue cracking/fracture
- Necessary corrective revision surgery
How Robins Kaplan Law Firm Can Help With Your Exactech Claim
Unfortunately, Exactech has not yet directly notified individuals who received the recalled knee or hip replacements. Instead, the company is relying on surgeons to inform their patients as to whether they are impacted by the defective medical devices.
Should your surgeon contact you about your device, please contact us to discuss your potential claim against the product manufacture, Exactech. You may be eligible to submit a claim if you or a loved one:
- Have an Exactech hip or knee implant, or
- Suffered side effects from the Exactech implant, or
- Received a letter or other communication from your surgeon informing you of the recall
Contact Us First
Contact the attorneys at Robins Kaplan first before contacting the Exactech-Broadspire Helpline. By signing or agreeing to compensation with a manufacturer, you may lose other rights to future claims and compensation. Contact a lawyer first.
If you were not yet contacted by your surgeon, Exactech’s online tool can help to determine whether your device is recalled. You will need to enter the serial number of your device, which can be found in your medical records. Exactech is a large medical device company located in Gainesville, Florida.
Our nationally recognized Mass Tort Attorneys assist clients who are injured by dangerous and defective products, and we are available to evaluate your potential claim against the manufacturers of Exactech.
Contact us for a free and confidential evaluation today. To find out if we can help you with a claim, call 1.800.811.9972 or complete our online contact form above.
RELATED PROFESSIONALS
Rayna E. Kessler
Partner
PUBLICATIONS
If you are interested in having us represent you, you should call us so we can determine whether the matter is one for which we are willing or able to accept professional responsibility. We will not make this determination by e-mail communication. The telephone numbers and addresses for our offices are listed on this page. We reserve the right to decline any representation. We may be required to decline representation if it would create a conflict of interest with our other clients.
By accepting these terms, you are confirming that you have read and understood this important notice.
